Ueki's Research Topics†
- Ueki's Research Topics
- 1. Ascidians – animals that concentrate the rare metal vanadium
- 2. What is ascidian?
- 3. What is vanadium?
- 4. Research overview
- 5. Research Topics
- Vanadium-binding protein
- Functional elucidation of redox reactions and metallochaperones
- Vanadium and proton and sulfate membrane transporters
- Genome, transcriptome, metagenome, commensal bacteria SDG14
- Development of highly selective metal separation and recovery technology using the selective metal binding ability of living organisms SDG12
- Adhesion mechanism and anti-fouling mechanism of ascidians: Elucidation of its dual nature and application
1. Ascidians – animals that concentrate the rare metal vanadium†
About 110 years ago, a German chemist Dr. Martin Henze visited Naples, Italy, and investigated the amount of metal contained in various marine animals at the marine station using the latest analytical methods at that time.
After collecting and analyzing a white fist-sized ascidian Phallusia mammillata, he discovered that the metal vanadium was abundant in the blood cells of this ascidian species (Photo: Gulf of Naples and Mount Vesuvius).
Since this discovery, many biologists and chemists have been interested in this phenomenon and have continued their research. The starting point for my research was the invitation of Professor Hitoshi Michibata, a pioneer in this field in Japan.
Professor Michibata has investigated the amount of metals such as vanadium contained in ascidians in various parts of Japan and around the world since the 1970s. As a result, we found that common edible ascidian Halocynthia species have almost no accumulation of vanadium, and that ascidians in the group Enterogona contain a large amount of vanadium. Humans contain only about 10 times as much as seawater, while ascidians accumulate at 100,000 to 10 million times the concentration.
2. What is ascidian?†
Ascidians are invertebrates that live mainly in shallow waters. Ascidians are relatively prosperous animals. Ascidians inhabit the sea just below the equator, both poles, and the deep sea, and about 3,000 species of ascidians have been identified so far. It has a very unique shape as shown in the photo (photo: Ascidia gemmata).
Ascidia gemmata is known to accumulate the highest level of vanadium as far as we have investigated, and its concentration coefficient of 10 million times is unparalleled in other organisms. Since this species did not have a Japanese name at that time, it was named "Vanadium Boya" after its characteristic concentration of vanadium.
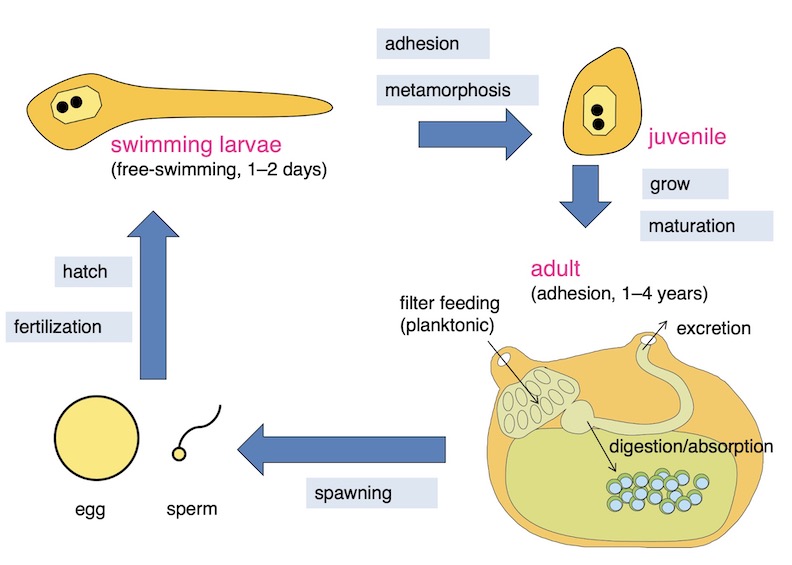
Immediately after hatching, the larvae become a tadpole-like form. After swimming for several days, they stick to rocks in seawater and metamorphose into adult forms. Adults have a lifespan of one to several years, during which they live in a adhesive type of life. Ascidians looks like mollusk shellfish, but taxonomically they are the same chordates as humans (photo: life cycle of sea squirts).
Research on molecular developmental biology and biological defense mechanism utilizing their characteristics, and analysis of the nervous system are active. Japan is a world leader in these studies. On the other hand, the accumulation of high concentrations of vanadium, the synthesis of cellulose, and the proliferation by budding are unique properties that are rarely seen in other animals, and their research is being actively conducted.
3. What is vanadium?†
Vanadium is a type of metal like iron and copper. Vanadium is an industrially valuable metal. Vanadium is used to make it strong by mixing it with iron, as a superconducting material, and as a catalyst for chemical reactions. Demand is also increasing for large secondary batteries used in solar power generation, wind power generation, etc.
Vanadium is also one of the essential trace elements and an element essential to living things. The concentration of vanadium in the human body is very small compared to iron, copper, etc., and research on vanadium has not progressed much.
On the other hand, vanadium concentration in sea squirts is extremely high, and research is progressing as an excellent model organism for elucidating the metabolic pathways and physiological functions of vanadium. On the other hand, Ishii et al. reported that the gill caps of Pseudopotamilla occelata, a type of annelid, also contain high concentrations of vanadium, and these are the only two groups of animals for which highly concentrated vanadium has been reported so far*1.
Vanadium is accumulated in the blood cells of sea squirts. The cells that contain vanadium are mainly vacuolated cells called "signet ring cells." The Vacuole makes up most of the cell, so the nucleus and cytoplasm are located in the periphery, and when viewed under a microscope, it has a ring-like shape (Photo: Blood cells of the A. sydneiensis samea. Arrows indicate signet ring cells)*2. In the case of P. occelata, it is accumulated in the vacuoles of the epidermal cells of the gill cap, which is different from that of the sea squirt.
4. Research overview†
Our research began with the highly accurate quantification of vanadium and chemical analysis of vanadium's existence patterns in the 1970s and 1980s by Michibata and Kanamori et al., followed by biochemical and cell biological research in the 1990s, and from the 1990s onwards. This progressed into molecular biological research through the 2000s. We have led the world by actively searching for the proteins and genes involved in each of the three mechanisms, the selective enrichment mechanism, the vanadium reduction mechanism, and the energy mechanism for concentration, and analyzing their functions. In particular, the novel vanadium-binding proteins Vanabins and VBPs that we discovered are a unique protein family possessed only by sea squirts that concentrate vanadium, and we believe that they hold the key to the concentration mechanism. Many related genes have also been isolated and functional analysis underway, and analysis of their roles and interrelationships has led to a complete elucidation of the vanadium enrichment mechanism by sea squirts.
It is not yet clear what physiological role concentrated vanadium plays. One possibility is its involvement in in vivo redox and electron transport mechanisms. Our research revealed that Vanabin has a reductase activity that reduces vanadium using the reducing power of NADPH. Experiments using EST analysis and microarrays have also found that gene expression of glutathione enzymes in the gills and gastrointestinal tract is associated with vanadium concentration, revealing the interrelationships between concentration, reduction, and energy mechanisms. Another possibility is a contribution to adhesion, a property unique to ascidians. We are proceeding with the analysis of microstructures related to adhesion and the search for adhesive substances. On the other hand, we have begun a comprehensive analysis of morphological changes and comprehensive gene expression changes when sea squirts are raised in a low-vanadium environment with the aim of exploring a wide range of possibilities.
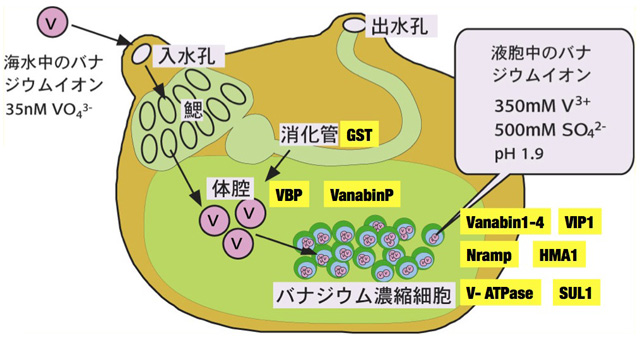
The concentration of vanadium in seawater is approximately 35nM. No mechanism has yet been discovered to directly incorporate vanadium at such low concentrations. Our research has revealed that bacteria living symbiotically in the gills and intestines concentrate and reduce vanadium.( We believe that these symbiotic bacteria may act as first-stage concentrators, helping the ascidian concentrate vanadium.
5. Research Topics†
Vanadium-binding protein†
We were the first in the world to clone the genes for vanadium-binding proteins (Vanabin1,2, 3,4*3, and P*4), which hold the key to the vanadium accumulation mechanism, from the Ascidia sydneiensis samea. We used this recombinant protein to determine the binding number and dissociation constant for vanadium(IV) ions.
Furthermore, by electron spin resonance method and NMR, we reported that vanadium ions bind to the site where lysine and arginine are localized (amine: R-NH2) in Vanabin2. In addition, we revealed that there are six types of Vanabin in A. sydneiensis samea*5, five types of vanabin in the Ciona intestinalis, and at least 2 types of vanabin in A. gemmata.
In addition, we found out about the duplication and variations in the Vanabin2 gene. We would like to focus on elucidating the physiological role of Vanabins and get to the bottom of the mechanism of high levels and highly selective accumulation.
Vanadium is taken up from seawater into the ceolomic fluid through the gills or the intestine, and is eventually accumulated in the vacuoles of a type of blood cell (vanadocyte). At this time, Vanabin1, 2, 3, and 4 present in the cytoplasm of vanadocytes are thought to act as intracellular metallochaperones, and VanabinP is thought to act as a metallochaperone responsible for transporting vanadium in the ceolomic fluid. We proceeded with biochemical analysis of metal transfer between Vanabins and interactions with other proteins, and succeeded in identifying a novel protein VIP1 that interacts with Vanabin. Although VIP1 is known to constitute a multigene family, its function is still unclear.
In addition, the vanadium binding protein extracted from the intetine of A. sydneiensis samea was glutathione transferase (GST)*6, which is involved in the accumulation and reduction of vanadium in the intestine, which is the first step in uptake from seawater.
Vanabin is a gene family that is found only in ascidians belonging to the order Phrebobranchia, which includes A. sydneiensis samea. No highly homologous genes have been found in ascidians that do not accumulate vanadium or in organisms other than ascidians. How ascidians came to have the Vanabin gene is still a mystery.
Functional elucidation of redox reactions and metallochaperones†
Through research using vanadocyte-specific antibodies, we discovered that vanadocytes specifically express a group of enzymes in the pentose phosphate pathway that produce NADPH. The pentose phosphate pathway is the pathway that produces the reducing substance NADPH. In recent research, we discovered that Vanabin is a novel vanadium reductase that reduces pentavalent vanadium to tetravalent vanadium through a cascade involving glutathione*7 and thioredoxin*8 based on the reducing power of NADPH. As far as we have investigated, this reduction reaction does not occur with metals other than vanadium*9, so we believe that this is the key to selective accumulation of vanadium.
Additionally, through microarray experiments using Ciona intestinalis, we found that gene expression of enzymes in glutathione pathway in the gills and intestine is related to vanadium concentration*10. This suggests that the glutathione system is actively involved in regulating the intracellular vanadium accumulation.
Vanadium and proton and sulfate membrane transporters†
In seawater, vanadium exists as a pentavalent anion V(V). Ascidians absorb V(V) from their gills and intestine, and ultimately reduce it to trivalent V(III) and accumulate it in the SRC vacuole. High concentrations of protons and sulfate ions are also accumulated in the vacuole. This makes sense since V(III) is only stable under strongly acidic reducing conditions. This phenomenon is common in ascidians with high vanadium concentrations, and there is a positive correlation between proton concentration and vanadium concentration in vanadocytes. Sulfate ion is considered to be necessary as a counter ion, and in fact, its valence and abundance ratio are reasonable. Similar ion accumulation has been confirmed in the vacuoles of the gill crown epithelium.
In general, the pH of intracellular organelles is actively controlled by vacuolar-type ATPase (V-ATPase). Immunohistochemical and molecular biology studies have revealed that the pH of the vacuole of ascidian vanadocytes is also controlled by V-ATPase. On the other hand, it was found that the Na(+)-dependent sulfate ion transporter of the Slc13 family is involved in the accumulation of sulfate ions. Ascidians, which are marine invertebrates, have a high Na(+) concentration in their blood cells, and a promising model uses this potential to actively transport sulfate ions from plasma into blood cells. The Km value of this transporter is 1.75 mM, and the sulfate ion concentration in plasma is 25 mM, so it can be said to have sufficient biochemical transport activity. The transporter that transports sulfate ions from the cytoplasm into the vacuole has not been identified.
As a transporter that transports vanadium, we revealed for the first time that Nramp, a membrane protein that transports divalent cations, transports vanadium through antiport with protons*11. Thus we showed that protons accumulated in the vacuole serve as the driving force for vanadium transport. The coupled transport and accumulation mechanism of these three ions is becoming clearer.
Genome, transcriptome, metagenome, commensal bacteria SDG14†
By using next-generation sequencers, it has become possible to decipher significantly large amounts of base sequence data. In joint research with the Okinawa Institute of Science and Technology Graduate University (OIST), we are proceeding with genome decoding of the ascidian, the transcriptome of the ascidian blood cells, and comparative metagenomic analysis between species and tissues of the ascidians. We are beginning to see results that complement previous research and help elucidate the overall picture.
We have isolated vanadium-resistant bacterial strains from the intestine of an ascidian. Two of these strains were found to highly concentrate vanadium*12. We are also getting data that they have the ability to reduce vanadium. Comparative metagenomic analysis also revealed bacterial strains that appear to be involved in vanadium accumulation*13. We have found bacteria in the gills of sea squirts that have the ability to take in low concentrations of vanadium in seawater. We would like to clarify how these symbiotic bacterial strains are involved in the uptake of vanadium by sea squirts and how they are related to the circulation of vanadium in seawater.
Development of highly selective metal separation and recovery technology using the selective metal binding ability of living organisms SDG12†
In order to realize a sustainable society, there is a need for technology that can stably obtain the necessary metals or remove harmful metals from industrial waste fluids. We found that when the Vanabin genes of the snail and vanadium were expressed in E. coli, they concentrated approximately 10 to 20 times more copper and 2 times more vanadium than the wild type*19. It is possible to use these E. coli strains as metal accumulators, but because they are genetically modified, there are limitations to their use in open environments. On the other hand, since the sea squirt intestinal bacteria mentioned above are bacteria that exist in nature, there are no restrictions on their use, and we believe that they could be used as metal accumulators in the future*14.
In fact, in order to put it into practical use, cost issues must be resolved. In addition to vanadium, various other bacteria have been reported, including bacteria that concentrate gold, bacteria that reduce gold, and bacteria that concentrate uranium, but it seems that there are still problems that need to be solved before they can be put to practical use.
Adhesion mechanism and anti-fouling mechanism of ascidians: Elucidation of its dual nature and application†
Ascidians firmly adhere to substrates such as rocks in seawater. On the other hand, many ascidians have a property that makes it difficult for other organisms to attach to the surface of their tunic. We are currently searching for the factors that give rise to these two contradictory properties*15. Regarding the adhesion prevention mechanism, research results have been reported that focused on the fine structure of the surface of the tunicate and the acid secreted by the tunicate*16. The results obtained may also be applied in the medical and industrial fields. In other words, this may lead to the development of new adhesive substances and biofouling prevention substances (antifouling coatings).
(under construction)